I felt under the weather couple days ago shivering, muscle ache, so I wanted to go ahead and try something different then taking medication and I’m glad I did. I used LPG followed with red light therapy and vibration plate. 2 days later I had no signs of feeling sickly. The owner Jessica walks you through the entire process with her knowledge. Spa was very clean and organized. No more taking pills and going through sickness for 2 weeks. I’m glad I found this place.
After researching several options for arm fat removal I decided to move foward with Jessica. She was awesome! Very knowledgeable and patient. I had my first CoolSculpting treatment last week and I am really excited to see the results! Definitely recommend this facility.
I'm so happy to find Embodyment by Nujo. Jessica has been so helpful with explaining all of the different treatments that they offer. She is super knowledgeable. I have really enjoyed LPG and the Red-Light Therapy and I'm looking forward to trying other treatments down the road. Give them a call you will not be sorry.
Jessica and Cori are great and very friendly.. They make you feel very comfortable and explain everything to you… Very nice and clean place
Great place to go to, the provide you with all the information you need. And they answer all your questions and concerns. I'll be recommending them to my friends and family.
Amazing atmosphere and amazing staff please take your time and come by you won’t regret it ❤️
⭐️⭐️⭐️⭐️⭐️ Jessica Rae and her team at Embodiment by Nujo are absolutely amazing! I came in for the Immunity IV, and the entire experience was quick, painless, and incredibly professional. They made sure I was comfortable the whole time, checking in on me and providing top-notch care. I highly recommend them and will definitely continue to use their services!
I had a BBL done and my doctor recommended me to do ENDERMOLOGIE 2 x weekly for 6 weeks, and today was my first session. I honestly could tell you that when I got there, I was barely able to move. After the session, I felt more flexible, and relieved. I honestly felt the difference and I’m excited to go back this Friday. Also through my whole session Jessica was awesome, and explaining everything she was doing. I definitely recommend this place you really feel the difference
Wide array of health & wellness options. Terrific staff! They get my highest recommendation!
I'm in love with jet plasma! I'm loving all their services the red light therapy is a game changer! Can't wait to try hyperbaric! everyone is so knowledgeable and so professional and this business is truly exceptional! I have done CoolSculpting there previously and my results were fantastic. Highly highly recommend!
In Westminster off pecos. After my red light treatment my muscles were so relaxed that my multiple sclerosis spasms were reduced significantly. I went to sleep that night very quickly, and had a great night's rest. The staff at Embodyment were outstanding and so helpful. They supported my heath goals by providing me a solution. I was impressed with the options that they provided as to preventative health treatments. Check out all the offerings on their website.
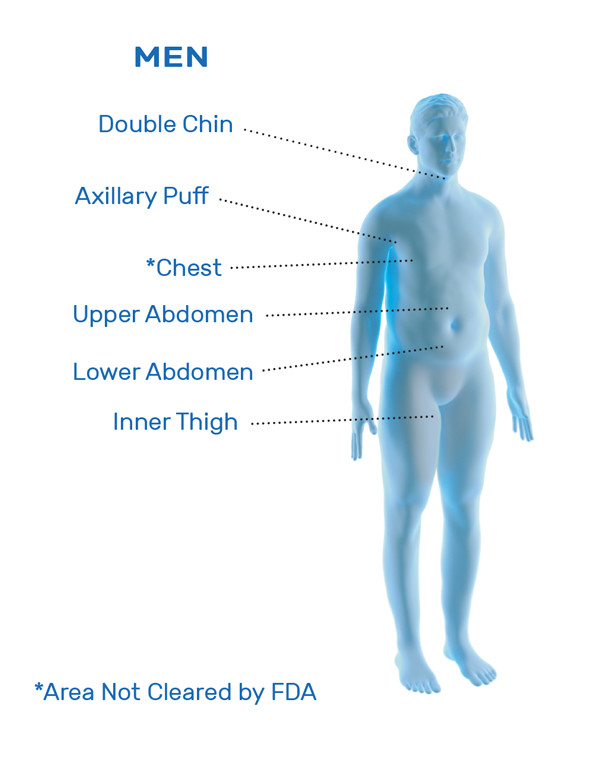
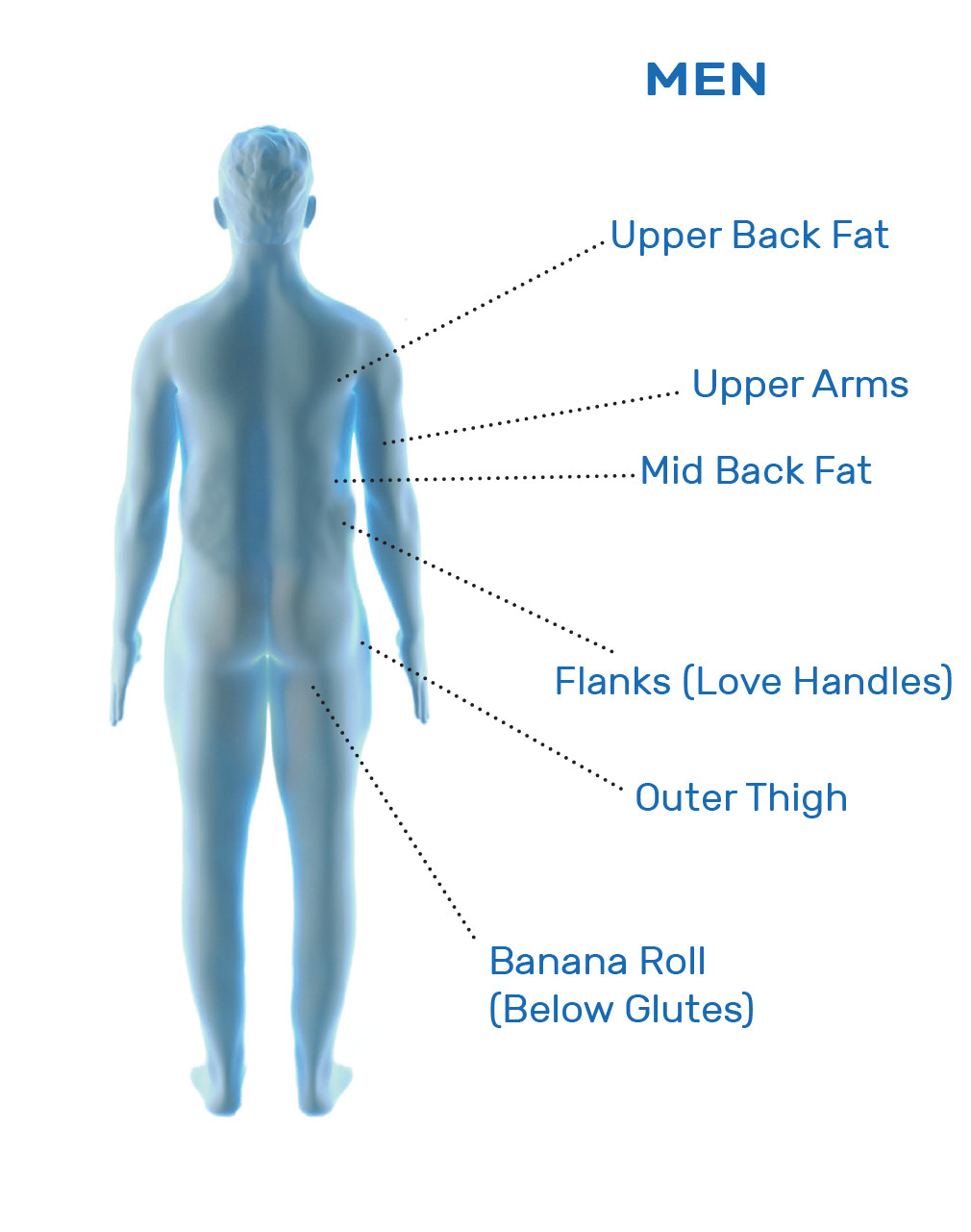
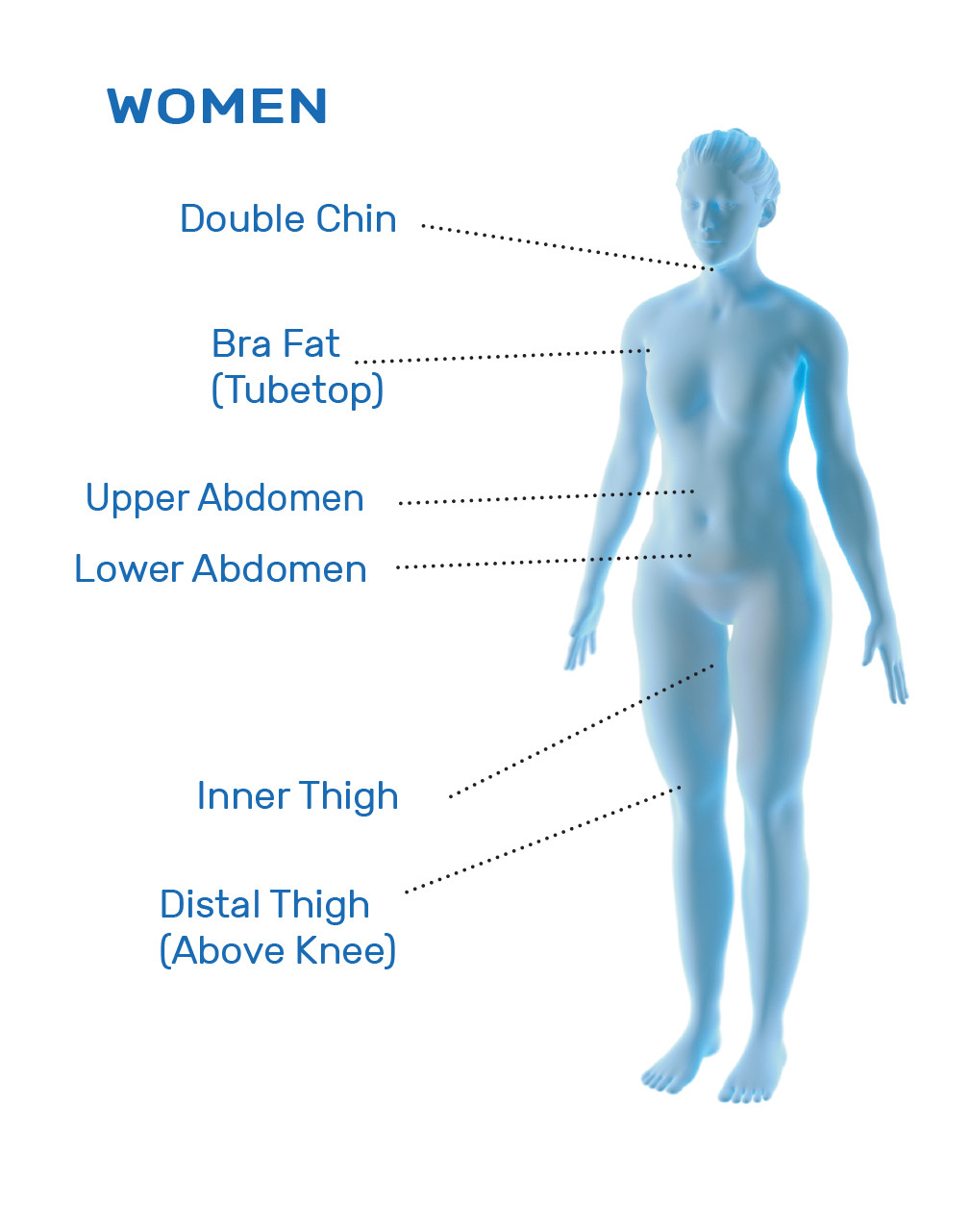
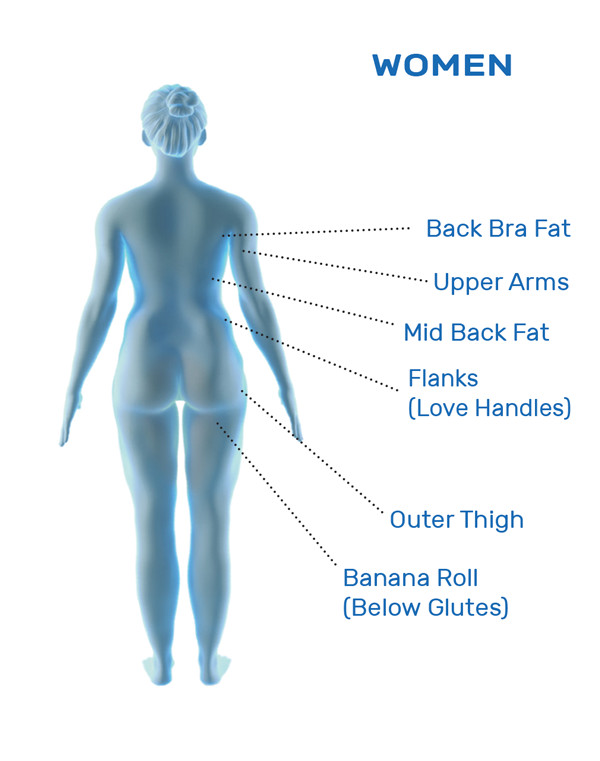
CoolSculpting® Uses
CoolSculpting® is FDA-cleared for the treatment of visible fat bulges in the submental (under the chin) and submandibular (under the jawline) areas, thigh, abdomen, and flank, along with bra fat, back fat, underneath the buttocks (also known as banana roll), and upper arm. It is also FDA-cleared to affect the appearance of lax tissue with submental area treatments. CoolSculpting® is not treatment for weight loss.
CoolSculpting® Important Safety Information
These procedures are not for everyone. You should not be treated with CoolSculpting® if you suffer from cryoglobulinemia, cold agglutinin disease, or paroxysmal cold hemoglobinuria.
Tell your doctor if you have any medical conditions including recent surgery, pre-existing hernia, and any known sensitivities or allergies.
During the procedure you may experience sensations of pulling, tugging, mild pinching, intense cold, tingling, stinging, aching, and cramping at the treatment site. These sensations subside as the area becomes numb. Following the procedure, typical side effects include temporary redness, swelling, blanching, bruising, firmness, tingling, stinging, tenderness, cramping, aching, itching, or skin sensitivity, and sensation of fullness in the back of the throat after submental or submandibular area treatment.
Rare side effects may also occur. CoolSculpting® may cause a visible enlargement in the treated area, which may develop 2 to 5 months after treatment and requires surgical intervention for correction.
Please see CoolSculpting® full Important Safety Information.
Patient Results May Vary.
Please view this link: Important Safety Information